Key Takeaways:
- The article details the surprising prevalence of oxygen's green glow in celestial observations, particularly in nebulae and auroras, which is caused by "forbidden radiation" emitted by oxygen atoms in near-vacuum conditions where excited electrons linger in a metastable state before releasing photons.
- The article discusses the historical misidentification of the green glow in nebulae as being caused by unknown elements ("nebulium") and the eventual understanding that it stems from oxygen, highlighting the challenges in early spectral analysis.
- The article explores the variability of human perception of color, particularly at low light levels, influencing the visibility of oxygen's green glow in astronomical observations through telescopes, emphasizing the differences between visual observation, photography, and spectroscopy.
- The article concludes by introducing the use of oxygen isotopes (O-16, O-17, O-18) as isotopic fingerprints to trace the origin of celestial bodies and their implications for understanding planetary formation, particularly in the context of the Moon's origin and the Giant-impact hypothesis.
Rewind to Mrs. Wombat’s sixth-grade science class. There we learned that the universe contains 92 natural elements. All also are found here on Earth. There’s no sense pretending they’re equally interesting. For example, every breath you take is a gassy mixture of 99.9 percent nitrogen, oxygen, and argon. Argon is inert and just floats there. We don’t use it for much of anything besides filling light bulbs. It doesn’t help us, and
it doesn’t hurt us.
Air also contains a smidgen of water vapor (H2O), which is two-thirds hydrogen. That’s the universe’s most common element, Mrs. Wombat insisted, and who were we to doubt her? Hydrogen makes up most of our brains. It’s the Sun’s primary fuel. It’s obviously crucial to our existence.
But now, in terms of cosmic abundance, consider element number two: helium. This takes us back to the not vital category. Our bodies contain exactly zero helium. If all of Earth’s helium suddenly vanished, most of us wouldn’t notice or care.
But give helium this: Inside stars, it undergoes fusion to create the universe’s third most abundant element, oxygen. Thus we’ve reached the hero of today’s adventure tale. Our lives critically depend on oxygen. It combines so eagerly with other elements that as we look around, either on Earth or through our telescopes, we see it almost everywhere. Although water may be two-thirds hydrogen in terms of its atomic makeup, it’s nearly 90 percent oxygen by weight. By the same yardstick, the rings of Saturn are mostly oxygen. So are clouds. And chocolate milk. Kittens.
Our blanket of air is 21 percent oxygen for just one reason: plants. They absorb oxygen in one of its combined forms (carbon dioxide), use the carbon to create their stiff, crunchy bodies, and then release molecular oxygen as waste. There are so many plants and trees and kelp, our air is thick with oxygen.
Nobody knew this at the start of the Renaissance. In fact, nobody knew that air is a mix of gases. But the hunt for knowledge was on. Scientists discovered air’s two major components almost simultaneously. Scottish physician Daniel Rutherford identified nitrogen in 1772; two years later, British theologian Joseph Priestly isolated oxygen. The elements’ main distinction was immediately obvious. One supported life and combustion; the other didn’t.
The big nonoxygen player soon acquired a ghoulish reputation. Rutherford called it “noxious air.” Mice placed in it quickly died. As for oxygen, it was the precious life-sustaining element everyone was then trying to detect. Because oxygen bonds so easily with most other elements, it makes up two-thirds of animal bodies by weight. And nearly half of the Moon. When wolves howl at the Full Moon, it’s basically oxygen calling out to oxygen.
In the beginning, the universe had no oxygen at all. The Big Bang created hydrogen, helium, and a little lithium in case any early stars suffered from depression. But no O. It appeared oh-so gradually starting perhaps 100 million years after the Big Bang — a pathetic seepage, really, trickling in after being forged in the unseen interiors of stars. Some of those early blue suns turned themselves inside out by violently blowing up and scattering their newly minted oxygen through the floorless alleyways of space.
British astrophysicist Arthur Eddington first figured out how the Sun and other stars shine in 1920. He correctly argued that the energy stems from the fusion of four hydrogen atoms into one new helium atom. As stars evolve into old age, they increasingly fuse helium to produce carbon and then oxygen. By the time a star like the Sun reaches the end of its life and collapses into a white dwarf, it’s a solid ball of oxygen and carbon and little else.
Before that crushdown unfolds, a typical star with up to about 8 solar masses sheds some material as an expanding gas bubble. This is where we as observers enter the picture. Whenever we look at a planetary nebula, we see an old-age central star surrounded by a glowing doughnut containing countless billions of tons of oxygen. Some of it — along with material from supernova blasts in even more massive stars — eventually helps form new stars and planets.
Like ours. Although, interestingly, we didn’t have an oxygen atmosphere until just 2.4 billion years ago. Then things started to get out of hand. A mere 300 million years ago, Earth was covered so thickly with plants that our air became superoxygenated. It reached a 35 percent concentration. This let evolution create a nightmare era of hypergiant insects. Some flies back then had wingspans that would rival today’s eagles. Try swatting them. Sometimes too much oxygen isn’t a good idea.
The green lantern
We’re observers, however, so the real trick is how oxygen gets us to pretty colors. (Actually, though it’s a colorless gas, oxygen liquefies into an attractive blue fluid.) In its gaseous form, oxygen usually doesn’t glow. Not when it’s cool. That’s because an atom can emit light only when an orbiting electron falls closer to the nucleus. Ordinary tranquil atoms, like those in the gases you’re breathing, are not being excited, so their electrons aren’t changing orbits and they’re not glowing.
Physicists were in an uproar. Every explanation proved to be incorrect. Early in the 20th century, German astronomer Julius Scheiner concluded that, “The auroral spectrum is absolutely identical with the cathode spectrum of nitrogen.” Beep, wrong! English meteorologist Marshall Watts was equally firm in an antithetical opinion that, “There seems now little doubt that the [glow of] the aurora must be assigned to krypton.” Beep! The wrong ideas flowed in torrents. Just a few years later, German spectroscopy expert Heinrich Kayser threw up his hands in exasperation: “We know nothing at all about the chemical origin of the lines of the polar light.”
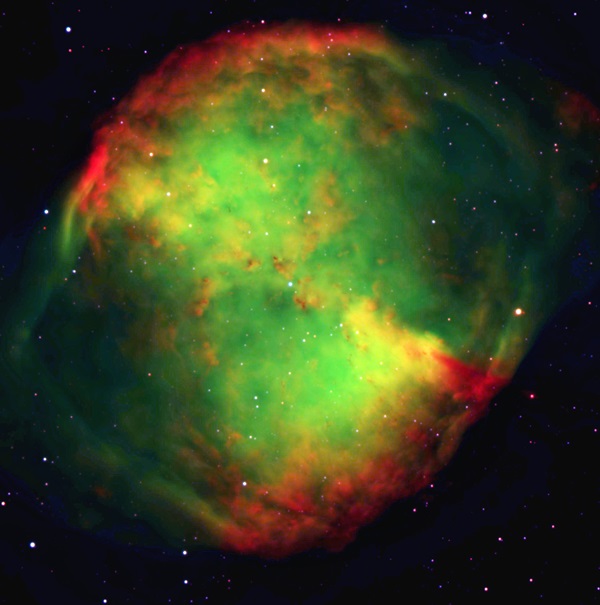
Still, everyone had an opinion. German researcher Alfred Wegener, soon to be famous for his theory of continental drift, published a major work on the atmosphere in which he suggested the auroral glow came from a new “geocoronium” gas. This notion of an undiscovered element producing a green light was not new. For nearly a century, scientists widely attributed the odd green glow of nebulae — which shine at an emerald wavelength of 500.7nm — to a substance called “nebulium.” The cosmos seemed awash with elements not found on Earth.
Decades passed. Lars Vegard, a Norwegian expert in the physics of the aurora, was sure he’d solved the green puzzle in 1924. As he wrote in Nature, “The typical auroral spectrum is emitted from solid [dust particles of] nitrogen.”
They were all wrong. As it turned out, the element nebulium does not exist, and neither does geocoronium. All along, the source of the green light was ordinary oxygen.
In both places — deep space and high in our atmosphere — the mystery arose from the near-vacuum conditions. You see, as Mrs. Wombat used to point out, oxygen’s electrons have certain allowable orbits around the nucleus. But when excited by solar electrons or a star’s high-energy ultraviolet radiation, the electrons jump to energetic yet unstable positions where they can’t stay. Near Earth’s surface where the air is thick, these excited atoms hit others so quickly that they dissipate their extra energy before they can emit it as light.
But in the rarefied upper atmosphere and also in the hard vacuum of deep space, oxygen’s electrons can linger in a “metastable” state before falling to a lower orbit and emitting photons. They thus give off colors never produced under more earthly conditions. This “forbidden radiation,” as it started to be called, comes in a precise shade of yellow-green at 557.7nm in the northern lights and blue-green at 500.7nm for planetary nebulae. (The latter emission originates from doubly ionized oxygen, which has lost two of its normal complement of electrons.) Oxygen. Nothing exotic, after all that trouble. Mystery solved at last.
Happily, these oxygen emissions happen just where the human eye is most sensitive. Still, in 2014 during my annual aurora tour to Alaska, which I’ve been conducting for decades, some of the 44 guests said they saw no color. To them, the aurora appeared pale white. I was startled because to my own eyes, the green was so intense that it seemed like a traffic light. So I took a vote. Result: Half the group saw the color as a rich green; one-quarter perceived it as pale green; and one-quarter saw no color at all. It thus became apparent once again that human eyes vary in their ability to see color at low light levels.
This individuality carries over to telescopic observations. Most people viewing the Orion Nebula (M42) through large backyard instruments — 10 inches in aperture and above — perceive a distinct green color. But not everyone does. Probably the most reliable nebulae that display rich green color are compact planetaries such as the Cat’s Eye Nebula (NGC 6543) in Draco and the Little Gem Nebula (NGC 6818) in Sagittarius. I used to lament that since most amateurs do not possess spectroscopes, they can’t fully examine and enjoy these hues. Recently, however, I’ve realized that when a celestial body emits most of its light in one narrow part of the spectrum — as planetaries do with their glowing oxygen at 500.7nm — you really don’t need a spectroscope. Your eye perceives accurate color through the eyepiece.
A spectroscope is most useful when observing mixtures of colors. Using one to look at Betelgeuse or Sirius is a wondrous experience because not only are all the emissions laid out side by side in dramatic fashion, but the differences between stellar spectral types also become beautifully striking. Betelgeuse shows numerous absorption lines, even some from molecules, whereas Sirius displays a simple series of sharp hydrogen lines overlaid on a vivid explosion of green, blue, and red. In contrast, a planetary nebula is a one-note composition with virtually all its light coming from that single green oxygen line.
Bottom line: What colors you see — and the issue of whether oxygen’s green glow dominates the scene — depends on your technique. Visually, oxygen rules. Photographically, it does not except in planetary nebulae. And spectroscopically, it’s a toss-up except again with planetaries. Through it all, and no matter what equipment you use, observing glowing oxygen is educational and fun.
The three wise isotopes
Oxygen also provides keys to critical celestial puzzles. That’s because it comes in three flavors. Oxygen has three stable isotopes, each of which has the same number of protons but a different number of neutrons in its nucleus. All have identical chemical properties, and you could happily breathe any of them. Every oxygen atom contains eight protons. The vast majority of these also have eight neutrons. Add up the protons and neutrons in this lightest-weight oxygen atom and you get 16, so this most common form is called O-16. But a small percentage of oxygen has one or two extra neutrons — O-17 and O-18, respectively.
The first stars in the universe created almost 100 percent pure O-16. Later generations made progressively larger proportions of the heavier isotopes. Nowadays, at least here in the solar system, about 1 in 500 oxygen atoms contains an extra neutron while about 1 in 2,000 have two extra. Still with us?
The ratios of O-16, O-17, and O-18 isotopes in every potato chip, puppy, and rock here on Earth follow a simple relationship. But if you send a lander to Mars and sample its material, as NASA has now done several times, you’d find that martian rocks with the same ratio of O-18 to O-16 as earthly specimens will have a slightly higher ratio of O-17 to O-16. The difference in the isotope ratio between our two planets is 300 parts per million, with Mars consistently higher.
So oxygen isotopes are like fingerprints. They tell you which world a rock came from. That’s how we can be sure a particular meteorite originated on the Red Planet. We also have meteorites from the large asteroid Vesta, whose ratio of O-17 to O-16 is about 300 parts per million lower than Earth’s.
Here’s where things get weird. The oxygen in Moon rocks has the same isotopic ratio as terrestrial objects. It’s as if the Moon is Earth! Any difference is less than 1 part in 50,000.
This presents a problem, however. Nearly every planetary scientist thinks the Moon resulted from a long-ago collision between Earth and a Mars-sized body dubbed Theia. The laws of physics show that for the impact hypothesis to work, much of the Moon should be Theia material and thus should have a distinct and alien oxygen-isotope ratio. Yet the Moon appears made of earthly stuff.
Every few years, some researchers will publish a journal article that either tries to explain this oxygen problem or else uses it to discredit the collision hypothesis. A major 2014 reanalysis of Apollo moon rocks claimed to find a minuscule disparity between our worlds’ oxygens — just barely enough, perhaps, to keep alive the idea of the Moon’s violent birth.
So take a deep breath. Ponder the element that energizes your brain so you can contemplate the cosmos. And someday, if we spot oxygen’s distinctive auroral glow on an exoplanet, the traffic-light green can serve as a “go” signal.
For, almost surely, we will have found the signature of plants on another world. It will be our beloved oxygen, once again guiding our discoveries.