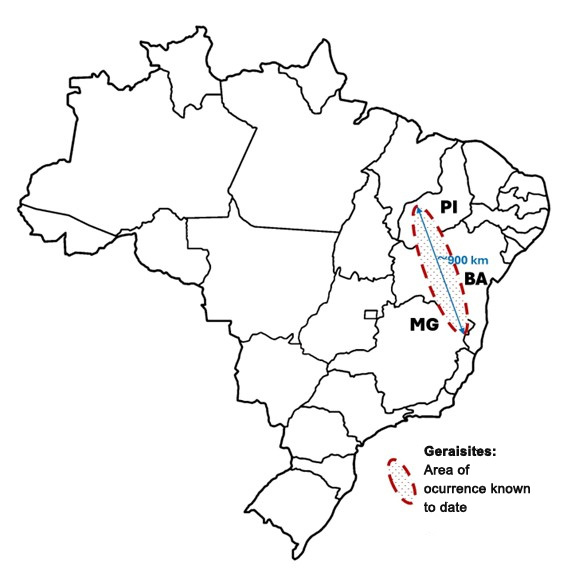
Sulfur is not terribly abundant in the universe. It shows up, however, near high-energy regions and objects such as supernova shock fronts and hot massive stars.
We typically see this element near areas where high-speed gases are colliding. The gaseous remains of exploded stars — called supernova remnants — tend to have relatively strong sulfur emission. We also see it in the gas ejected from dying Sun-like stars and their remnants, planetary nebulae. Hot young stars emit ultraviolet light, which kicks electrons off nearby atoms, thus ionizing them. Sulfur is one of the ionized gases we see near these young suns. Sulfur is useful to astronomers as a diagnostic tool that shows us where shocked gases and high amounts of ultraviolet radiation are.
Thus, to capture images of such regions, the Hubble Space Telescope’s Wide Field Planetary Camera 2 (1993–2009) included a narrowband sulfur filter. This filter let only a small range of light around a wavelength of 672 nanometers pass through to the camera.
Pennsylvania State University,
University Park