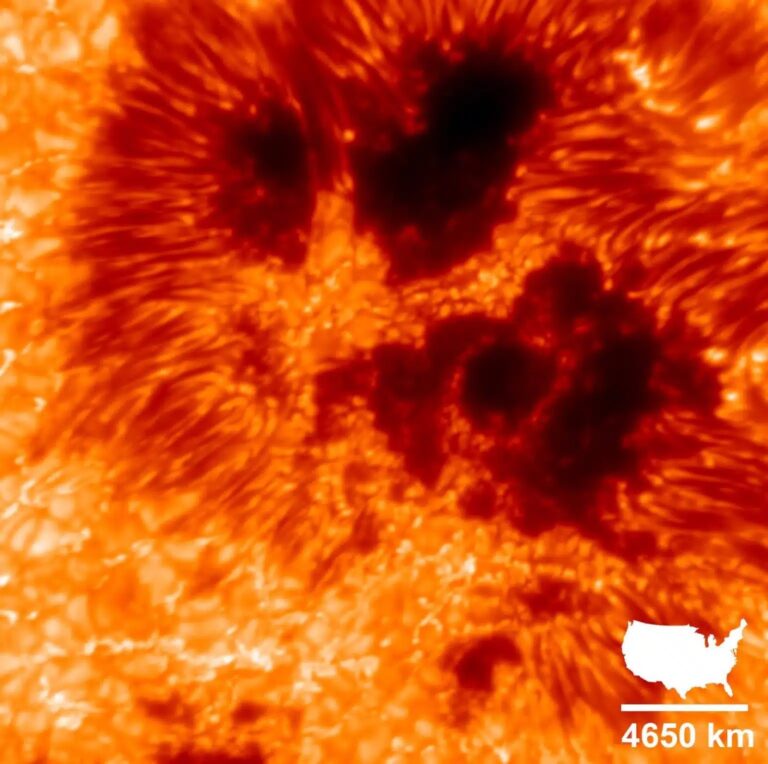
Use a really long rope, and drop your bucket roughly 432,000 miles (696,000 kilometers) down to the Sun’s center, where the density is about 5.4 pounds per cubic inch (162 grams per cubic centimeter) — 14 times that of solid lead on Earth. If you could preserve a sample (which you can’t), a standard 2.5-gallon bucket brought back to the lab would weigh 3,384 pounds (1,535 kilograms). The most remarkable thing is that it’s still a gas! The reason is the temperature. You’d need a very well-insulated rope because temperatures start at 9940° Fahrenheit (5500° Celsius) at the Sun’s surface and climb to a whopping 28.3 million degrees F (15.7 million degrees C) at the center, which is high enough to keep the solar interior gaseous.
At the surface, nearly 91 percent of the stuff in the bucket would be hydrogen, while nearly 9 percent (by number of atoms) would be helium, which is pretty much the average ratio throughout the cosmos. That’s close to 100 percent, so it doesn’t leave much room for other chemical elements. The third most abundant, oxygen, constitutes just less than 0.045 percent, iron a bit less than 0.003 percent, and lead 5 billionths of a percent. Your bucket from the Sun’s surface would weigh just 4.2 millionths of a pound (1.9 millionths of a kilogram).
For the past 4.6 billion years, the inner 20–25 percent of the Sun (by radius) has been fusing hydrogen into helium with a slight mass loss that, via Albert Einstein’s famous equation, E=mc2, is converted into the energy that fights against gravity to keep the Sun from contracting. (That mass loss is also what we see and feel as sunlight.) Your heavy bucket from the solar center would now contain about as many helium atoms as hydrogen.
Come back in another 5 billion or so years, and the core will look quite different: The gas will be nearly all helium, and the energy production will have shut down (temporarily), allowing gravity to take over for a while. Then the Sun will swell outward to become a red giant. The contracting at the core will lead to higher density and thus temperature, allowing helium to fuse into carbon and oxygen. Then the Sun will lose all of its outer layers, leaving behind the core as a white dwarf. Your bucket dipped to an average depth within the final white dwarf would contain hardly any hydrogen or helium at all, and it would hold some 21 million pounds (9.5 million kg) of material.
University of Illinois,
Urbana-Champaign