Key Takeaways:
- On Earth, hydrogen exists in solid, liquid, or gaseous states, but remains a non-metal due to its tightly bound electrons.
- Within Jupiter and Saturn, extreme pressure and temperature transform hydrogen into liquid metallic hydrogen, characterized by unbound electrons facilitating electrical conductivity.
- This metallic hydrogen is hypothesized to be the primary driver of Jupiter and Saturn's magnetic fields, unlike Earth's iron-based dynamo.
- Uranus and Neptune, despite containing some hydrogen, lack the necessary internal pressure and temperature for the formation of metallic hydrogen.
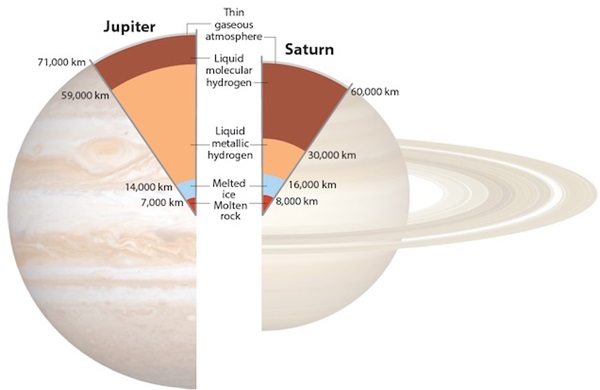
Our solar system has two gas giants: Jupiter and Saturn. Both planets contain a significant percentage of hydrogen, based on their densities. But at the temperatures and pressures deep inside these giants, their hydrogen becomes so heated and compressed that it enters several strange states, including liquid metallic hydrogen. As I mentioned earlier, hydrogen is a non-metal. But inside Jupiter and Saturn, hydrogen atoms at high temperatures and pressures actually lose their electrons, creating a free-floating stew of hydrogen nuclei (protons) and electrons. Because the electrons are unbound, they can move easily between the nuclei — a property associated with metals. This is metallic hydrogen: hydrogen that behaves like a metal. Metallic hydrogen is conductive, and it’s believed to be largely responsible for the dynamo that powers Jupiter’s and Saturn’s magnetic fields. (Whereas on Earth, that dynamo is powered by liquid iron, an actual metal.)
Uranus and Neptune — our solar system’s ice giants — are too dense for hydrogen to be a major component of their makeup. Planetary scientists estimate that hydrogen makes up only about 15 percent of their masses, and furthermore assume that the interiors of these two planets are roughly the same because their masses are so similar. While hydrogen does exist in the ice giants’ atmospheres, and is also believed to form a liquid molecular shell deeper down, the hydrogen inside Uranus and Neptune is never subjected to the temperatures and pressures required to reach a metallic state.