How do scientists determine the chemical compositions of the planets and stars?
Cristina Montes
Muntinlupa, Philippines
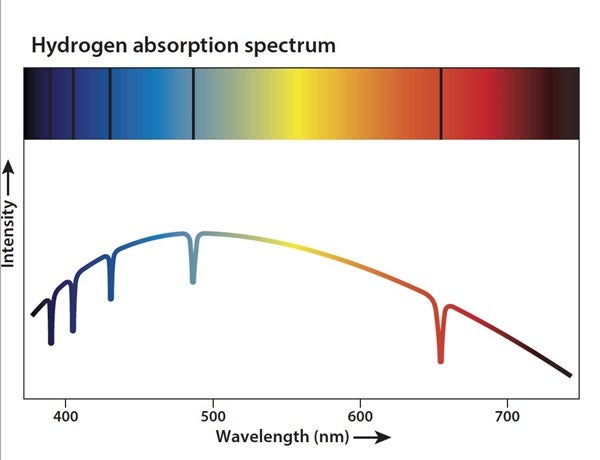
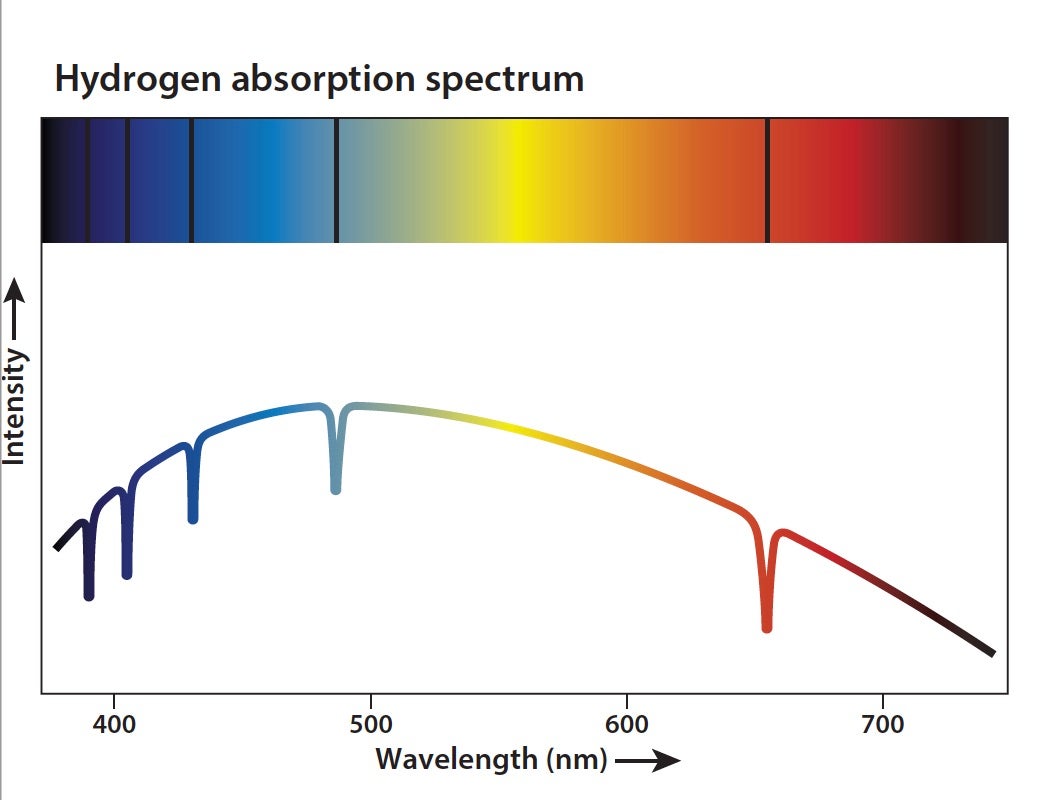
The most common method astronomers use to determine the composition of stars, planets, and other objects is spectroscopy. Today, this process uses instruments with a grating that spreads out the light from an object by wavelength. This spread-out light is called a spectrum. Every element — and combination of elements — has a unique fingerprint that astronomers can look for in the spectrum of a given object. Identifying those fingerprints allows researchers to determine what it is made of.
That fingerprint often appears as the absorption of light. Every atom has electrons, and these electrons like to stay in their lowest-energy configuration. But when photons carrying energy hit an electron, they can boost it to higher energy levels. This is absorption, and each element’s electrons absorb light at specific wavelengths (i.e., energies) related to the difference between energy levels in that atom. But the electrons want to return to their original levels, so they don’t hold onto the energy for long. When they emit the energy, they release photons with exactly the same wavelengths of light that were absorbed in the first place. An electron can release this light in any direction, so most of the light is emitted in directions away from our line of sight. Therefore, a dark line appears in the spectrum at that particular wavelength.
Because the wavelengths at which absorption lines occur are unique for each element, astronomers can measure the position of the lines to determine which elements are present in a target. The amount of light that is absorbed can also provide information about how much of each element is present.
The more elements an object contains, the more complicated its spectrum can become. Other factors, such as motion, can affect the positions of spectral lines, though not the spacing between the lines from a given element. Fortunately, computer modeling allows researchers to tell many different elements and compounds apart even in a crowded spectrum, and to identify lines that appear shifted due to motion.