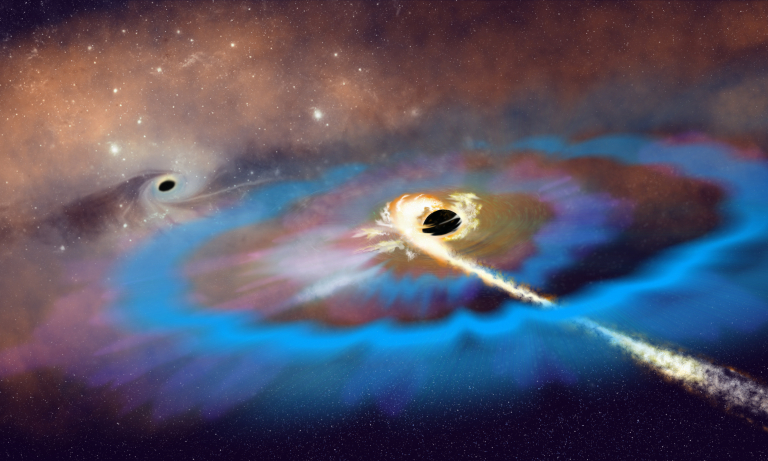
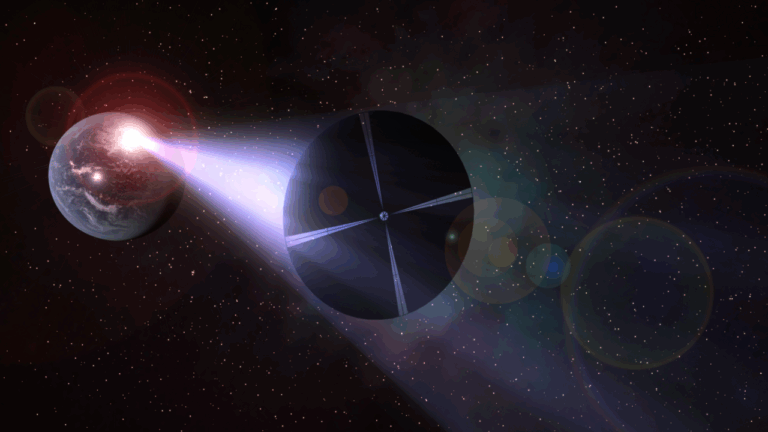
In a remote galaxy, two neutron stars circled one another in a ballet of ultimate destruction and inevitable creation. Both objects were the remnants of massive stars, probably from a binary system, that had become supernovae long before. Each was incredibly massive, with neutrons so closely packed that their cores became diamond. The dance, alas, could not go on forever and the stars collided, releasing unimaginable energy and sending gravitational waves speeding through the fabric of space-time.
In 2017, 1.3 billion years later, astronomers detected those waves with the Laser Interferometer Gravitational-wave Observatory. Albert Einstein’s prediction that the universe should be filled with such faint ripples caused by gravity from massive objects included sources such as neutron star mergers. Yet finding a disturbance in the fabric of space-time from this kind of event had proven elusive until then. When news of the detection of gravitational waves broke, the media wanted to know what else happens when neutron stars collide. Astronomers explained that, beyond the destruction of the stars and the ripples in space, such events also create all the heavy elements we know in the blink of an eye. But what did the media key into? That gold comes from outer space.
Gold-plated history
It’s not surprising that of the many elements formed in the cataclysmic destruction of massive stars, gold should be the one that captures our imaginations the most.
Elements necessary for life, such as carbon, oxygen, potassium, and sulfur, should rank higher on a list of favorites. But we have an emotional connection with gold.
Thousands of years ago, someone may have seen a shiny object in a stream and picked up a piece of gold. It must have looked intriguing — though, because the metal is so soft, it’s not very useful. Archaeologists have found a 6,500-year-old gold bead in Bulgaria and recovered a nearly 3,000-year-old gold coin from the Black Sea. The oldest known gold artifacts in England were found buried at Stonehenge, part of the grave goods belonging to a mysterious individual from Europe.
Gold was treasured, though useful only as decoration or for trade, and not much else. But its scarcity made it desirable, and its unchanging nature made it alluring: Unlike silver, which turns black, copper that goes green, or iron that rusts, gold never changes. It seems to be immortal — a gift from the gods.
It took 20th-century science to unravel this mystery. Iron, silver, and copper rust or turn colors due to reactions with oxygen. Oxygen is always hungry for electrons. Iron will give up two or three electrons to oxygen and will oxidize (rust) as a result. Other elements also fall victim to oxygen. But not gold. It’s the most nonreactive of all metals because it refuses to share electrons with oxygen.
Elemental facts
Like all the heavy elements on the periodic table, there just isn’t much gold to be found. If all the gold mined in human history were formed into one solid cube, it would measure about 70 feet (21.3 meters) on a side. That would be around 183,000 tons of gold. Sounds like a lot, but if melted, it would fill only three and a half Olympic-size swimming pools. In 2018, Barrick Gold Corporation’s mines in Nevada processed millions of tons of ore to recover just 4 million ounces (125 tons) of gold.
Because it is so dense and heavy, most of Earth’s gold sank to the core of our planet. Australian geologist Bernard Wood estimates that 99 percent of the world’s gold is buried thousands of miles below our feet. He also estimates that 1.6 quadrillion tons of gold lie within the core. Wood calculates that all this gold, if brought to the surface, would form a layer of the shimmering metal just 16 inches (40.6 centimeters) thick. Compared to Earth’s total size, that’s not much gold. There is actually six times more platinum in our planet’s core, which contains about 1 part per million of gold. Gold is, in fact, quite rare.
Our golden Sun
Working one night in 1859, chemists Robert Bunsen and Gustav Kirchhoff saw a fire raging in the town of Mannheim, Germany, about 10 miles (16 km) from their Heidelberg University laboratory. They rolled their newly improved spectroscope (a device they invented that breaks light into its component wavelengths, which allows chemical elements to be identified) to the window and quickly detected the elements barium and strontium within the bright glow given off by the flames. Bunsen wrote that the “same mode of analysis must be applicable to the atmospheres of the Sun and the bright stars.” The second half of the 19th century saw an explosion of discoveries using this powerful tool.
During the total solar eclipse on August 18, 1868, several astronomers using spectroscopy detected a new element — and, it turned out, the universe’s second most abundant one — in the Sun: helium. Carbon, nitrogen, iron, and all the heavier elements of the periodic table — including gold — were eventually identified in a gaseous state in the Sun’s atmosphere.
In the late 18th and early 19th centuries, rock and mineral collecting became the science of geology. Men and women such as Charles Lyell, James Hutton, and the great fossil collector Mary Anning, discoverer of the first Ichthyosaurus skeleton, clearly demonstrated that Earth was far older than the 6,000 years suggested by many contemporary theologians. Lyell and Hutton said Earth must be millions or even billions of years old. If this was true, what could keep the Sun and stars shining for such an incredibly long time?
German physicist Julius Robert Mayer strongly favored the meteoric theory of solar heat. He had calculated that, lacking an external source of energy, the Sun could shine for only 5,000 years. In 1848, he suggested the Sun was fueled by billions of meteorites raining down on it, which provided its energy. This material also, supposedly, would have brought heavy elements to our star.
Eventually, scientists calculated that the Sun contains almost 2.5 trillion tons of gold, enough to fill Earth’s oceans and more. Still, that’s just eight atoms of gold for every trillion atoms of hydrogen — a tiny amount when compared to the mass of the Sun. But how did gold come to be in the Sun and Earth?
Science is golden
For more than a millennium, alchemists struggled to transmute one element into another. They were in search of the philosopher’s stone, which could turn base metals like lead and mercury into gold. Even the great Isaac Newton was fascinated by the idea of transmutation. Indeed, some historians refer to him as “the last great alchemist.” But the tremendous forces of nature that create the elements were beyond the grasp of these early experimenters.
The origins of heavy elements started to come into focus with the publication of Einstein’s special theory of relativity in 1905. It was in this seminal work that the equation E=mc2 first appeared. It wasn’t obvious at first just how important this equation was to our understanding of the universe, but its application to the problem of the Sun’s immense energy output would have far-reaching implications. Not only did it explain why the Sun and other stars could shine for billions of years, but it also helped show how elements heavier than hydrogen form.
Almost two decades after Eddington and others began to explore fusion, German-American physicist Hans Bethe described the now famous proton-proton chain reaction that explains how hydrogen fuses into helium. Deep within the Sun is a vast “soup” of hydrogen atoms consisting of one proton and one electron each, which are in constant, rapid motion. Most of the time, the electromagnetic force repels any collisions. Trying to stick similar poles of two magnets together gives a feel for such repulsion. But collisions happen, and protons fuse together. When four protons eventually fuse, helium–4 forms, releasing energy and making the Sun shine.
Our Sun contains enough hydrogen to continue this fusion process for another 5 billion years. Eventually, helium will begin to fuse, forming the final products of carbon, nitrogen, and oxygen (element No. 8). Within more massive stars, whose stronger gravity creates more pressure and heat, elements beyond oxygen can fuse. But this process can continue only until iron (element No. 26) forms at the center of giant stars, and that’s when fusion shuts down. Finally, the star’s core will collapse and then rebound in a supernova explosion.
Once astronomers had pinpointed the source of the 2017 gravitational waves, researchers at the Max Planck Institute for Astronomy were able to detect strontium in the maelstrom of matter expanding into space at nearly 30 percent the speed of light. This element and others were formed by the rapid neutron capture reaction. The merger of these stars sent 1022 free neutrons flying through just 1 cubic centimeter of space every second.
Such a high density of neutrons creates conditions that allow existing elements to quickly capture free neutrons. Strontium, thorium, uranium, and even gold form in a literal flash. And off they go into the depths of space. During the nearly 14-billion-year life span of our universe, this has happened enough times to seed the nebulae that eventually collapse to form solar systems such as ours with gold — and all the other heavy elements, too.
Gold standard
Gold pervades our lives. The element is in every cellphone and computer. We coat sunglasses and astronaut visors with it. Gold thread is used in electronics and in clothing. Nations pay their debts with gold. We make precious objects out of it, from jewelry to religious artifacts. We put gold in our teeth and even make toilets with it. Doctors inject patients with gold to help relieve rheumatoid arthritis. You can even eat chocolate covered in gold.
Carl Sagan famously said we are made from the stuff of stars. So is the world around us. The next time you glance at the gold ring on your finger or feel the gold chain around your neck, remember that they are indeed a gift from the stars.