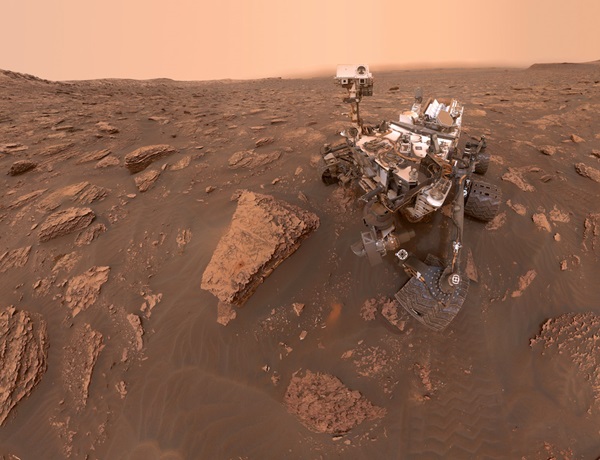
First, let’s get the most important question out of the way: No, life has not been found on Mars. That being said, Curiosity has measured the total organic carbon present in rocks on the Red Planet for the first time.
Before we delve into the results themselves, let’s answer the obvious question: What is organic carbon? As its name suggests, organic carbon is a key molecular component for all known forms of life. “Total organic carbon is one of several measurements [or indices] that help us understand how much material is available as feedstock for prebiotic chemistry and potentially biology,” said Jennifer Stern of NASA’s Goddard Space Flight Center in a NASA press release. In other words, the total organic carbon in an area can tell scientists how much raw material there was available to supply any potential life on Mars.
On Mars, “We found at least 200 to 273 parts per million of organic carbon,” said Stern. “This is comparable to or even more than the amount found in rocks in very low-life places on Earth, such as parts of the Atacama Desert in South America, and more than has been detected in Mars meteorites.”
A mixed result
This isn’t the first-time organic carbon has been found on Mars. Another Curiosity result published earlier this year showed that several rock samples were rich in organic carbon. But the new result is the first time the total amount of organic carbon was measured on the Red Planet.
To take the measurement, Curiosity drilled into rocks at a geological formation named Yellowknife Bay in Gale Crater. The location was chosen as the rover’s landing site because it was once home to an ancient martian lake.
The rocks themselves are mudstone, formed when very fine sediment settled at the bottom of a lake and was buried. They are an estimated 3.5-billion years old. Using the onboard Sample Analysis at Mars (SAM) instrument, Curiosity baked the rock. Then, by sprinkling in some oxygen, the organic carbon was converted into carbon dioxide (CO2).
This resource-intensive experiment has only been performed once by Curiosity in its nearly 10 years on Mars.
But while organic carbon is the basis for all (known) forms of life, it isn’t necessarily a smoking gun, as it could be delivered to Mars by non-biological means. Meteorites, volcanoes, and even reactions on the martian surface could all be responsible for creating the organic carbon.
To help clarify the possible sources of the organic carbon, SAM also measured the carbon isotopes present in the sample, providing key information on where the element may have come from.
Isotopes are kind of like different flavors of an element. Each isotope has a different number of neutrons — and, therefore, weight — allowing scientists to distinguish them from one another. Carbon-12 is lighter than carbon-13, for example. And because being lighter means that it’s quicker to react, life tends to prefer to use carbon-12.
But as Stern pointed out, the presence of carbon-12 doesn’t necessarily mean life definitely once existed on Mars. “In this case, the isotopic composition can really only tell us what portion of the total carbon is organic carbon and what portion is mineral carbon,” she said. “While biology cannot be completely ruled out, isotopes cannot really be used to support a biological origin for this carbon, either, because the range overlaps with igneous (volcanic) carbon and meteoritic organic material, which are most likely to be the source of this organic carbon.”
Nonetheless, it is promising that organic carbon was found on Mars yet again, especially in quantities similar to what we see in certain places on Earth. While the Red Planet is unlikely to currently be home to a thriving microbial population, the planet looked much different billions of years ago. Mars once has a more Earth-like climate and water that flowed into rivers and seas, the evidence of which we can still see today.
Dehydrating Mars
Curiosity has been exploring Gale Crater for nearly 10 years now. And in the past year, the rover has entered a so-called transition zone, where a clay-rich region is giving way to one filled with sulfate, a salty mineral.
Originally explored as a way to deepen our understanding of Mars’ watery past, the team is now finding this zone to also include a billion-year-old record of the Red Planet’s shifting climate. Curiosity has tracked the streams that once flowed through the area drying into trickles.
“We no longer see the lake deposits that we saw for years…” said Ashwin Vasavada, Curiosity’s project scientist at NASA’s Jet Propulsion Laboratory in Southern California, in another press release. “Instead, we see lots of evidence of drier climates, like dry dunes that occasionally had streams running around them. That’s a big change from the lakes that persisted for perhaps millions of years before.”
Soon Curiosity will take its last rock sample from this zone, providing even more data on the rich mineral composition of ancient Mars. And perhaps it will add to the list of reasons why scientists think Gale Crater may have once been a haven for life. Besides having water and organic carbon, the region was low in acidity, had chemical energy sources, and other elements needed for life, like oxygen, nitrogen, and sulfur.
As Stern explained: “Basically, this location would have offered a habitable environment for life, if it ever was present.”