They are 99.9999999999995% empty space. Almost perfectly nothing.
And yet they make up everything you see, touch, smell, taste, and feel. They feed and clothe you. Their motion makes you warm (and cold). They generate your hopes and dreams and memories. They exist in splendid isolation and in highly complex assemblages. They tell time. And they can reveal secrets of the past that are otherwise unknowable.
They are atoms.
You’ve likely never seen an atom, even though they permeate your world. This is unsurprising when you realize that they are not just mostly emptiness, they are very, very tiny pieces of emptiness — it takes 15 million trillion of them to create a single poppy seed. If atoms were the size of marbles, the poppy seed’s atoms would fill a warehouse 20 stories tall the size of New York State.
Yet astonishingly, we have learned how to engage directly with these bits of near nothingness so that we can ask them intimate questions, tweak their internal states, and read their complex histories. They can answer deep questions about the past. How did human diets change through time, and when did we develop crops that led us to abandon nomadic life? When, and why, did the dinosaurs suddenly disappear after 180 million years of predominance? How did life arise so quickly after Earth’s formation and why are its key molecules all left-handed? When in the history of the Universe was the gold in my wedding band forged?
By probing, counting, exciting, and transforming our little atomic historians, detailed answers to these and many other questions await us. But first, we require a more formal introduction.
We now know an atom’s constituent parts in exquisite detail — a complex, positively charged nucleus made of protons and neutrons (themselves composed of yet more fundamental entities we call quarks), orbited by negative electrons that belong to another category of particles dubbed leptons. Indeed, even our picture of these constituents as “particles” – bits of stuff in a given spot, moving at a particular speed – has fallen away, replaced by the non-intuitive quantum chimera of particle-waves.
But ignoring this complexity for the moment, we have re-established the notion, first posited in ancient India and Greece, that the smallest unit of any substance is the atom (or a precisely prescribed combination of them).
Furthermore, we now know that the history of the Universe and all it contains is written in the particular arrangements of the fundamental atomic constituents —leptons and quarks — that comprise the building blocks of all the normal matter in the cosmos today. Given an understanding of the physical laws that govern the behavior of these particles, we can read that history, just as given the laws of grammar and syntax we can read the documents historians have written. Although atoms are not culturally biased as human historians can be, they do have biases of their own, and we will have to be watchful for those as we cross-examine our atoms to extract their historical revelations.
As noted above, atoms are tiny — trillions can dance on the head of a pin without stepping on each other’s toes, even more impressive than angels. Their internal structure is, indeed, an elaborate minuet of charged particles, and the rhythm of their dance can be used to identify them across billions of light-years of space. Remarkably, the atoms we see out there are identical to those of which we are made.
What do these tiny bits of near emptiness look like? Well, if I placed a tennis ball outside my office at the corner of 120th Street and Broadway in Manhattan to represent a Hydrogen atom’s nucleus (the simplest kind), its electron would be orbiting somewhere between 96th Street and 145th Street roughly two kilometers away; walking at a brisk pace, it would take you half an hour to get there. And what would you see? Most likely nothing since 1) the electron, on this scale, is far smaller than a grain of sand (at least one hundred thousand times smaller, actually), and 2) it would be whizzing around at about 1350 miles per second — a smeared out cloud of evanescent probability that you’d be very unlikely to catch at all.
This book entails a whirlwind journey — a quantitative history of the Universe over the past 13.8 billion years — told through a series of tales, always with atoms in the starring roles. As we shall see, there are 94 leading actors in our drama known as the elements, and to give them their due, their names will be capitalized throughout.
The remarkable stability of these atoms, and the unique signatures each provides to the careful observer, allow us to reveal the entire story in impressive detail. We can use atoms to assign precise dates to works of human creativity, to trace the history of agriculture and human diet, to piece together the vicissitudes of past climate as an aid in understanding what our future might hold, and to reconstruct the history of our Solar System and the Universe itself. We will uncover art forgeries, identify the provenance of stolen statues, and determine the causes of death of ancient fellow humans (and what they ate for lunch the day they died). We will deduce the Earth’s temperature 100,000 years ago and relate it to the composition of the atmosphere at that time. We will date the formation of our planet and its moon and mark the origin of life on our calendar. With our exquisite understanding of the atomic world we can, quite literally, reconstruct history atom by atom.
Chapter 15: Our Sun’s Birthday
My paternal grandmother was from Belarus and had no idea when her birthday was. This was not a consequence of any loss of mental faculties – she was sharp as a tack up to her death at 99. It’s just that it wasn’t very important if you were a late nineteenth century peasant in Czarist Russia to remember the day you were born.
I suppose the same could be said about the Solar System – figuring out its exact birthday isn’t a burning issue of great import. But it would satisfy an idle curiosity. And such a history might reveal the processes by which the planets came to be arranged the way they are, while extending the reach of our atomic historians beyond the lifetime of Earth itself.
Several different types of meteorites fall to Earth, their composition and structure reflecting their different origins. Iron meteorites which are at least 95% Iron, Nickel, and Cobalt come from large bodies (large asteroids or protoplanets that didn’t quite make it to planethood before being broken up in a collision); these bodies had sufficient mass to become molten, and the heavy metals sank to the center (this segregation of the elements by mass is called differentiation and is what is responsible for the Earth’s rocky crust and Iron core). Stony meteorites are, as their names suggest, like rocks, composed mostly of Silicon- and Aluminum-based minerals; some of these are pure stones and thus are likely unprocessed from the time of their original formation, while others are mixed with some metal and may have come from differentiated asteroids.
The most primitive, least processed meteorites making up just under 5% of the total that reach Earth are the Carbonaceous Chondrites. Some of these contain a significant amount of water (from a few percent up to more than 20%), implying they cannot have been heated to high temperatures since their formation or the water would have evaporated into space. Many of these are also rich in organic compounds including amino acids. Of most interest for our current project, however, is that such bodies contain chondrules and CAIs, the earliest solid compounds to form in the early solar nebula.
Chondrules are small globs of minerals ranging in size from a hundredth of a millimeter to 1 cm across. They are primarily composed of Silicon and Oxygen with admixtures of varying amounts of Aluminum, Magnesium, Potassium, Calcium, Phosphorus, Chromium and other such elements between number 11 (Sodium) and number 26 (Iron) in the Periodic Table. They are believed to be the remnants of dust grains in the solar nebula that were quickly (in minutes) melted when heated to a temperature of ~1000 K. The source of this flash heating is uncertain, but could include solar flares, shock waves in the rotating disk of material in which they were embedded, collisions with larger bodies, etc. Following the heating, they quickly recondensed to solid form and were accreted by large bodies and incorporated into the rocky matrix of, say, an asteroid. Some chondrites are glassy (the molecules are jumbled together in an irregular pattern) while others are crystalline (where the molecules form a highly regular lattice).
CAI stands for Calcium- and Aluminum-rich Inclusions that are found only in carbonaceous chondrite meteorites. They are similar to chondrules but apparently formed at even higher temperatures (>1300 K); whether they are systematically even older than chondrules has been a matter of debate, although recent evidence suggests similar ages dating from the first ~1 million years of the Solar System’s history. They consist of various minerals such as anorthite (CaSi2Al2O8), perovskite (CaTiO3) and forsterite (Mg2SiO4) among many others.
To date something as old as the Solar System requires radioactive isotopes with long half-lives (a billion years or more). In addition, we cannot use the simple accumulation clock (Ch. 6) in which one radioactive isotope decays to a stable daughter isotope unless we know how much of the daughter was there to start with; the lack of geologists standing by as the Solar System formed puts us in a bind. But a clever technique using isochrones (literally “equal times”) gets us out of this dilemma. Figure 15.1 shows the Rubidium-Strontium isochrone which has been used to date chondrules as well as many rocks from the Earth and Moon.
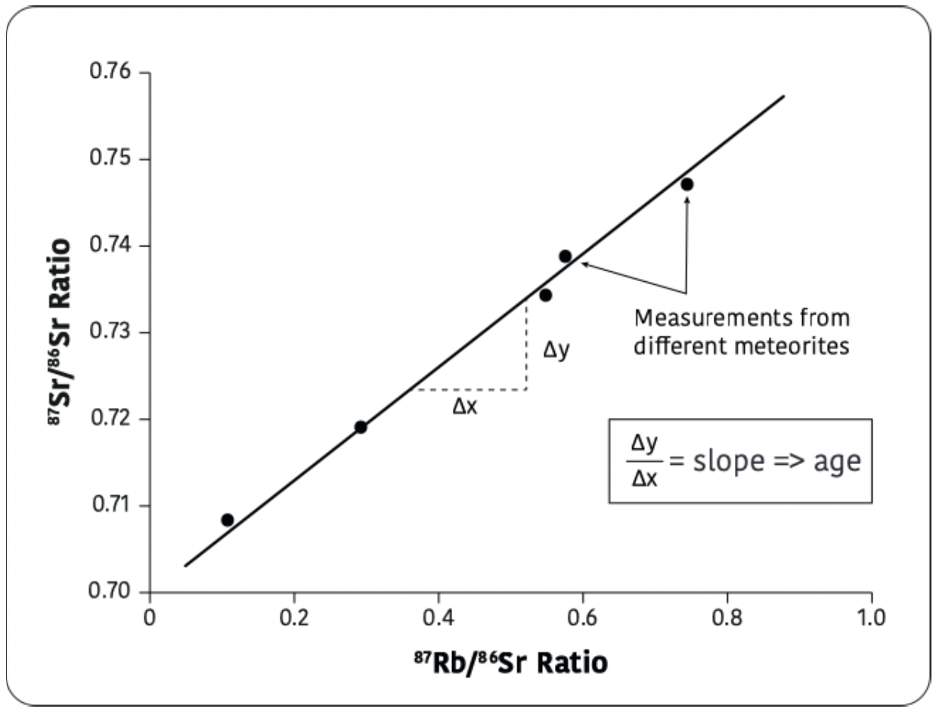
Rubidum-87 (87Rb) undergoes a standard beta decay to Strontium-87 (87Sr) by spitting out an electron and an antineutrino; the half-life is 49 billion years. The problem is, 87Sr is already present in some unknown quantity in the sample before the radiogenic atoms of this isotope start appearing from the Rb decay. To solve for this unknown, we also measure the amount of 86Sr, a stable, non-radiogenic isotope, in the sample, and construct the plot shown; a little algebra shows that the slope of the line gives the age.
Given the long half-life of Rb, less that 9% has decayed since the beginning of the Solar System. But 238Uranium has a half-life almost perfectly matched to the time we are trying to measure: 4.5 billion years. For the meteoritic chondrules and CAIs, the most accurate dates have been derived from an analogous isochrone technique called Lead-Lead dating.
Recall that 238U decays to 206Pb, and 235U decays to 207Pb. In addition, there is a non-radiogenic isotope of Lead, 204Pb. By plotting 207Pb/206Pb vs 204Pb/206Pb (and taking into account very slight variations in the initial 235U/238U ratios in the solar nebula), one can construct isochrones to obtain an age for a CAI from the Efremovka meteorite of 4,567.35 +/-0.28 Million years ago, while chondrules from other meteorites range from 4,567.32 to 4,564.71Mya. Besides being an easy number to remember (4,5,6,7 Mya) this suggests that CAIs and the earliest chondrules formed at the same time, though the latter perhaps had an extended period formation over 2 or 3 million years. The accuracy of this date is noteworthy: an uncertainty of 0.28 Myr out of a total of 4,567 Myr is equivalent to your being able to tell my age to within 1.5 days, a feat not possible with or without radioactive dating!
Our atomic historians thus allow us to reconstruct history back to the first years of the Solar System’s existence. Over the past two decades our discovery of thousands of extrasolar planets orbiting their own stars marks this history as less than extraordinary. It is, nonetheless, our history, and dating its beginning so precisely is gratifying. Now it is time to turn to the history of the historians themselves to see how the particular arrangements of the leptons and quarks that make up our world came to be.
Excerpted from The Universal Timekeepers by David Helfand, published by Columbia University Press. Copyright (c) 2023 David J. Helfand. Used by arrangement with the Publisher. All rights reserved.
You can order your copy of The Universal Timekeepers: Reconstructing History Atom by Atom here.