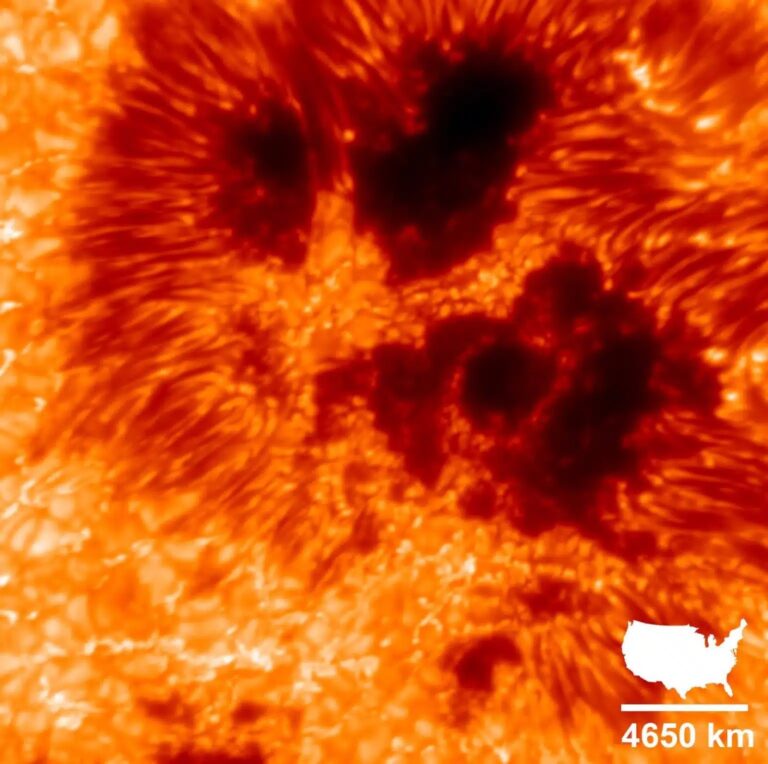
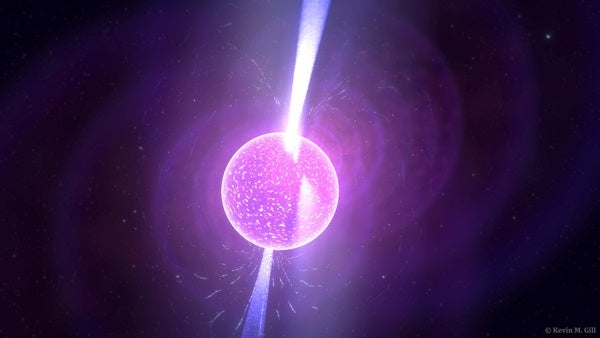
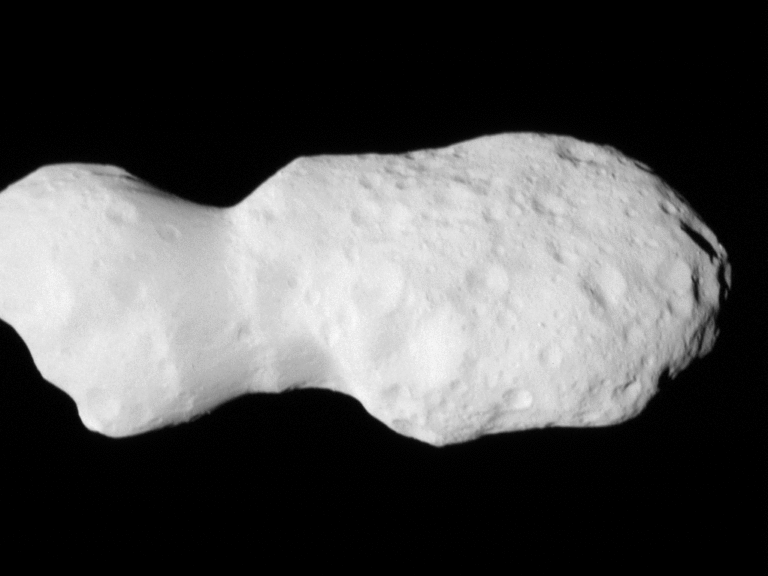
The answer to this question stems from Albert Einstein’s ideas. The equation E=mc2 states that mass (m) and energy (E) are manifestations of the same thing; mass can change into energy, and energy can change into mass. The speed of light (c) is the “conversion factor” between the two quantities. According to this equation, photons don’t have energy at all because their masses are zero, but this equation doesn’t really apply to light.
The full version of this Einsteinian equation, though, does: E2=m2c4+p2c2. The first part is zero because m is zero, so we’re left with E=pc, where p is momentum. Although photons don’t have mass, they do have momentum because they are moving (fast!). The higher a light wave’s frequency is, the more momentum it has and the more energy it carries, just as larger masses equal higher energies. Gamma rays have higher frequencies than radio waves, so they have higher energies.
The full version of this Einsteinian equation, though, does: E2=m2c4+p2c2. The first part is zero because m is zero, so we’re left with E=pc, where p is momentum. Although photons don’t have mass, they do have momentum because they are moving (fast!). The higher a light wave’s frequency is, the more momentum it has and the more energy it carries, just as larger masses equal higher energies. Gamma rays have higher frequencies than radio waves, so they have higher energies.
Sarah Scoles
Associate Editor
Associate Editor