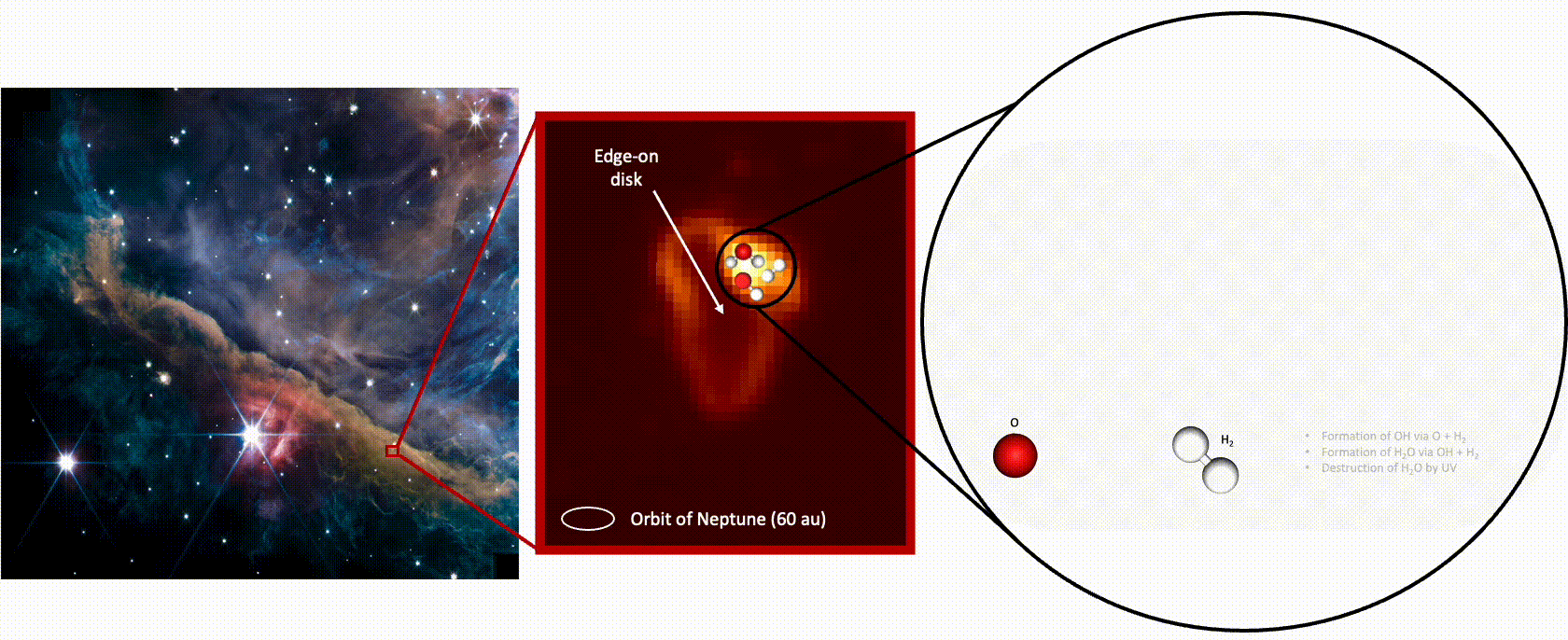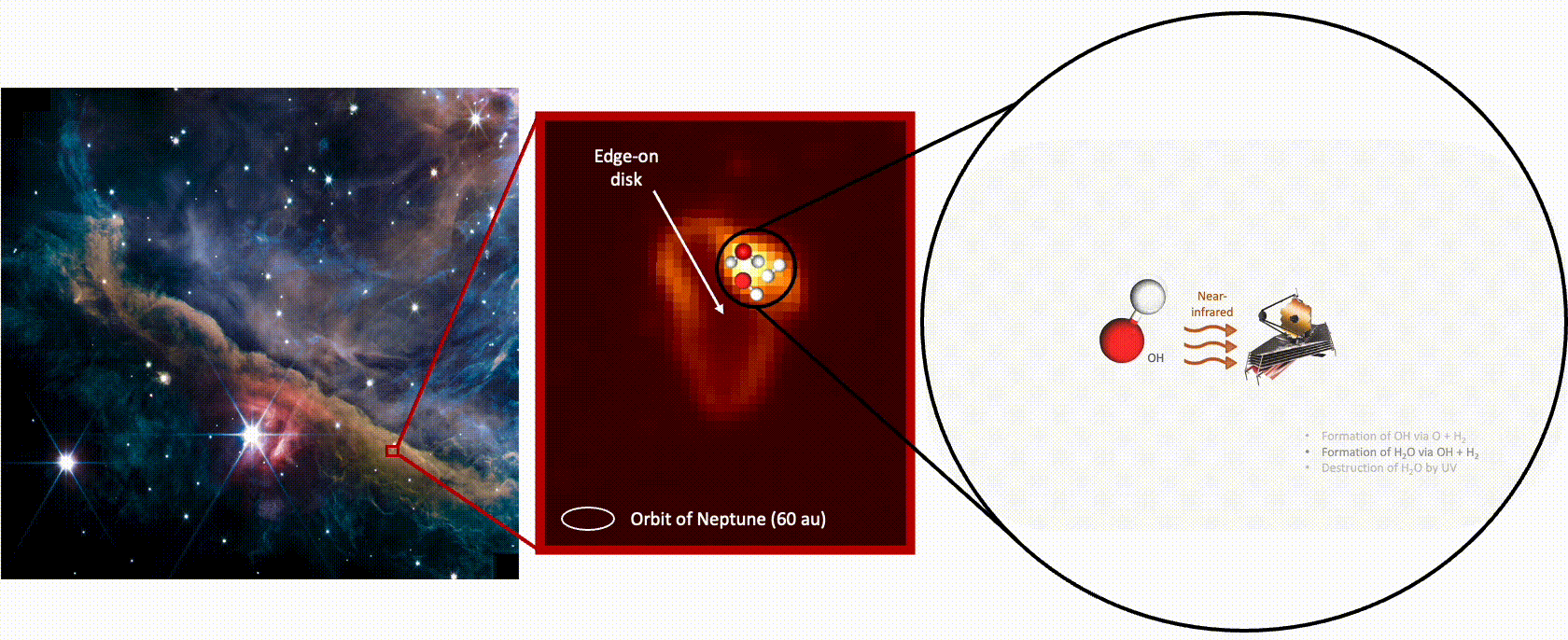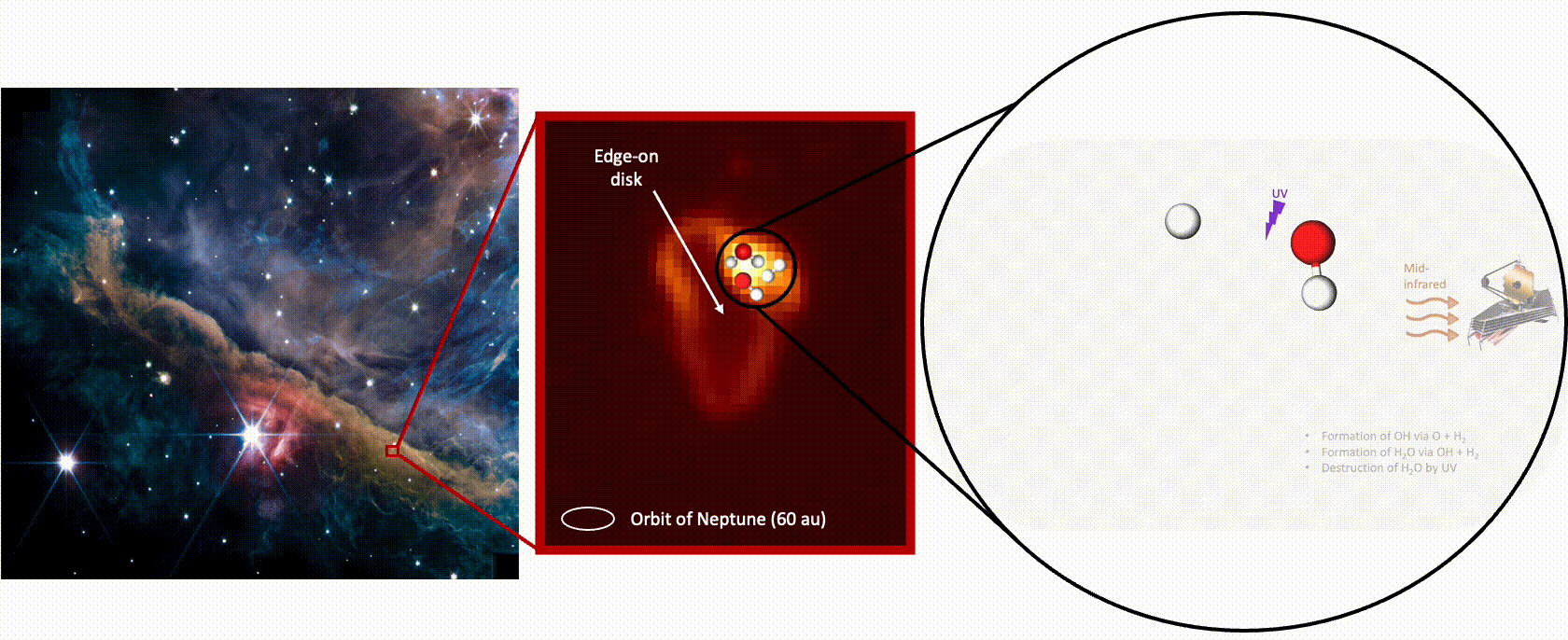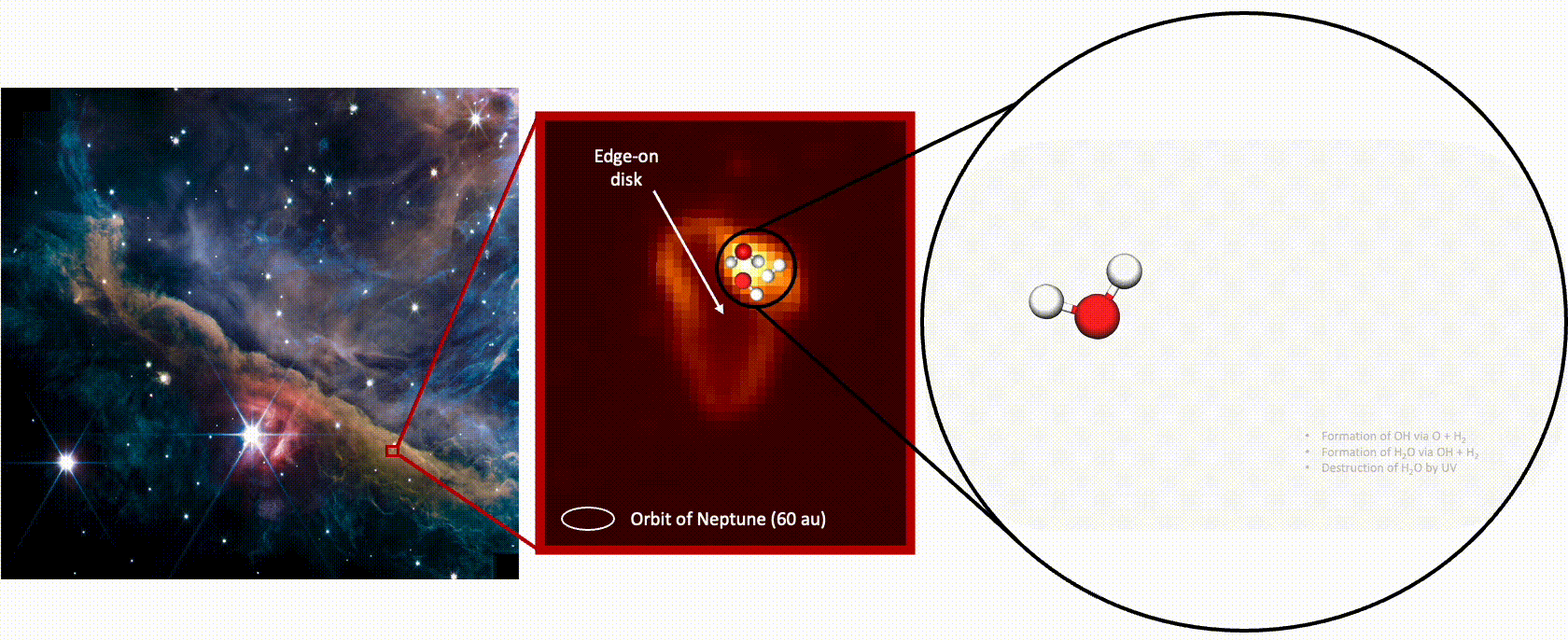
In the warm, dusty disk called d203-506 around a young star in the constellation Orion, oceans’ worth of water is being destroyed and replenished every month, new observations from the James Webb Space Telescope (JWST) reveal.
Because water is vital for life — along with energy, bioessential elements like carbon, and favorable climatic conditions — knowing where water exists and in what abundance helps astronomers determine the potential habitability of planets.
Building a water budget
The latest observations of d203-506, which lies more than 1,000 light-years from Earth, show how water molecules are destroyed and re-formed in the conditions inside planet-forming disks. The recurring cycle makes water molecules “lighter” by decreasing the quantity of deuterium, an isotope of hydrogen sensitive to temperature. (“Normal” hydrogen has only a proton and an electron, while deuterium also contains a neutron.) In addition to helping scientists understand how water evolves before being integrated into planets, the latest findings could also explain the composition of Earth’s oceans, which have low amounts of deuterium compared to those found around very young stars.
“It’s a lot of very sound and strong observational work,” says Manasvi Lingam, an astrobiologist at the Florida Institute of Technology, who was not involved with the study, which was published Feb. 23 in Nature Astronomy. Although it’s impossible to determine the amount of water that will eventually be part of planets that may or may not coalesce in this disk, the work has determined the available “water budget” in the system, he says, which is an important step toward a better understanding of planetary evolution.
A pressure cooker
The protoplanetary disk d203-506 can be likened to a pressure cooker, says Benoît Tabone, a researcher at the University of Paris-Saclay in France and a co-author of the new study. Hydrogen gas, warmed by ultraviolet (UV) radiation from a nearby cluster of massive stars, is escaping this disk at such incredible speed that the entire disk should evaporate within a million years. The escaped gas envelopes the disk as a thick “atmosphere” — not unlike the one blanketing Earth — revealing the disk within as a silhouette and preventing even the most powerful telescopes from peeking inside. The incessant stellar radiation has a potential upside, though: Once infused with radiation, which scientists think is 100,000 times more intense than that from our own Sun, the gas emits in infrared wavelengths, making the region a perfect target for JWST.
The new findings were collected as part of the PDRs4All program, an international collaboration coordinated by a core team of 20 scientists dedicated to observing pockets of the cosmos infused with UV radiation from massive stars.
Using JWST, “we’re able to detect molecules with so much precision that we actually need experts in other areas to understand in its entirety the phenomenon and mechanisms that we’re observing,” says Marion Zannese, a graduate student at the University of Paris-Saclay and the study’s lead author.
Detecting the full cycle of water in this region required two of the telescope’s instruments. The Mid-Infrared Instrument (MIRI) captured the destruction of water by capturing the photons emitted from hydroxyl radicals — made up of a hydrogen and an oxygen atom — created when UV radiation destroys (or photodisassociates) a water molecule, knocking off one of its oxygen atoms. The hydroxyl radicals are left “rotating very, very fast almost to the point where the molecule could break up,” says Zannese.
“When we detect a photon that is coming from a rotating OH [hydroxyl radical], it means that a water molecule has just been photodisassociated,” explains Tabone. “By counting the number of photons emitted, you count the number of water molecules that are being photodisassociated.”
That count, combined with computer models incorporating the known intensity of the UV radiation from the nearby stars, revealed the huge volume of water — oceans’ worth — being evaporated from the disk every month.
Meanwhile, data from JWST’s Near Infrared Spectrograph (NIRSpec) detected another signal from the hydroxyl radicals, which suggests they then fuse with a hydrogen atom to form a water molecule — the familiar H2O, says Zannese. And the NIRSpec measurements suggest this is occurring at a higher rate than the speed with hich water is being destroyed.
All that water may not ultimately be integrated into planets, though, because it has been found in gas that’s being depleted from the disk. “But there might be water getting reprocessed the same way in a part of the disk that we can’t probe,” says Zannese.
A peek into Earth’s past
It’s hard to tell just how many times water molecules were broken apart in the past, although it appears that it takes one to two days for the cycle to unfold, according to Tabone. “The water that we’re looking at has probably been destroyed and re-formed thousands of times,” he says.
The reprocessed water “is not formed in the same conditions as water at the beginning,” adds Zannese. The disk’s initial water molecules likely formed as ices on the surfaces of miniscule dust grains in temperatures as low as –418 degrees Fahrenheit (–250 degrees Celsius). That water would have been enriched in deuterium, whose amount diminishes with warmer conditions, such as those as observed in d203-506. In this way, the water cycle ultimately changes the composition of water in that region, says Zannese.
A similar process likely occurred in our own solar system’s past. While most of Earth’s water formed in frigid conditions that existed long before the Sun’s birth, scientists think a fraction of this water evaporated and re-formed at higher temperatures within the protoplanetary disk surrounding our young Sun, just as is happening in d203-506 now.
In the coming months, Tabone, Zannese, and the PDRs4All team plan to further probe the complex chemistry of the region by studying additional data taken by JWST during its observations, which includes a myriad of other molecules.
“We really want to continue studying these small, excited molecules and get everything we can out of them,” says Zannese.