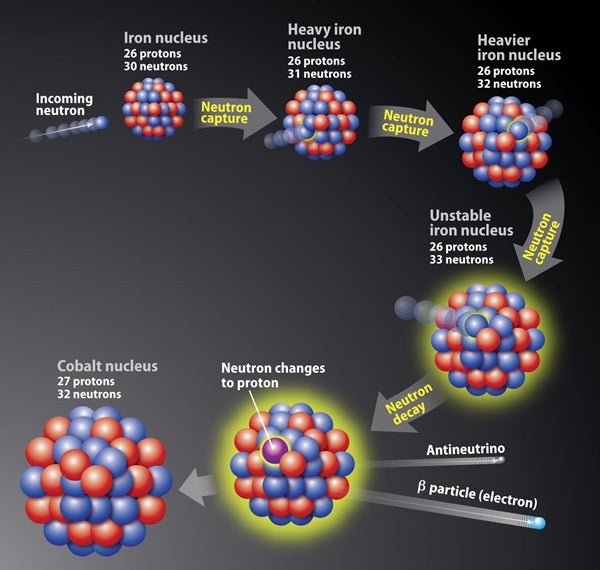
In certain environments (such as a supernova), an iron nucleus can capture neutrons. Eventually, one of those neutrons will convert into a proton, an electron, and an antineutrino. This process, called “neutron capture,” then changes the iron nucleus (26 protons) to a cobalt nucleus (27 protons). These events repeat to create heavier elements.
Astronomy: Roen Kelly
In an environment packed with excess energy (like a supernova), a small fraction of this energy may be used to bind free neutrons to iron atoms. One of the neutrons in the nucleus decays (or changes) into a proton, and the nucleus’s charge increases by one to produce a heavier element.