This story comes from our special January 2021 issue, “The Beginning and the End of the Universe.” Click here to purchase the full issue.
The question “Are we alone?” has long permeated our collective psyche. As early as the second century A.D., humankind was recording stories of aliens and space travel: Lucian of Samosata’s A True Story features a war between the inhabitants of the Sun and the Moon. And a simple look at ancient mythology tells us that humankind has wondered what might exist among the stars for far longer.
With modern instruments, astronomers have discovered more than 4,000 planets orbiting other stars, and many of these exoplanets are far more exotic than we could have imagined. What kind of life could exist on a world with two or three suns? Or a world made of diamond? How about one where it rains glass? The universe is a really, really big place, so the possibilities are almost endless.
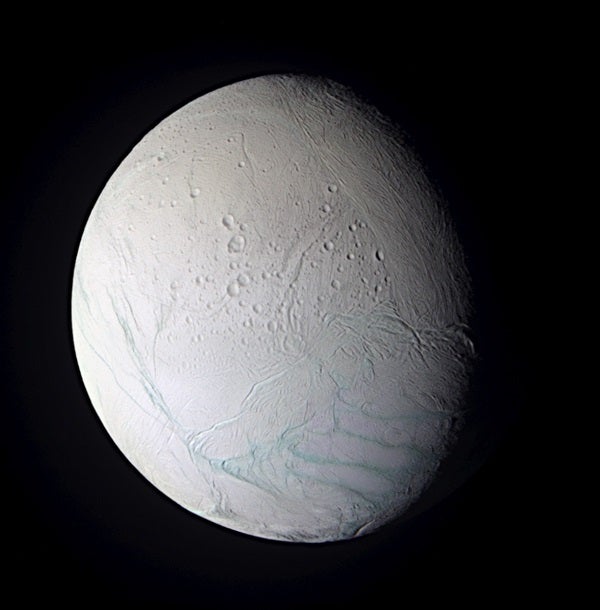
Before we go too far down the rabbit hole, there are many worlds that appear Earth-like — meaning they’re in a stable orbit around a G–type star and they sit in the star’s habitable zone, where liquid water can exist on the planet’s surface. Scientists have even found multiple ocean worlds in our own solar system, such as Jupiter’s and Saturn’s moons Europa and Enceladus, which both hide oceans beneath their icy shells.
Still, the universe is strange, and, as Dr. Malcolm says in Jurassic Park, “Life, uh, finds a way.” If science has learned one thing from science fiction, it’s that extraterrestrial life could be beyond even our wildest dreams. But life still has to follow some basic rules.
The chemical necessities
Life is likely to rely on locally available building blocks for parts; scientists don’t expect life to be based on an element that’s extremely rare, such as iridium or platinum. When searching for life in the cosmos, astronomers tend to look for what is most probable (and detectable). That means homing in on chemical signatures containing the 10 most abundant elements in the observable universe: hydrogen, helium, oxygen, carbon, neon, iron, nitrogen, silicon, magnesium, and sulfur.
But that’s not the whole story. For example, silicon is more common on Earth than carbon, and yet all life on Earth is carbon based, meaning that carbon forms the scaffolding for other elements to be built upon. While silicon is somewhat similar to carbon in terms of its elemental makeup, it is different in important ways. For instance, at Earth’s temperature, carbon dioxide (CO2) is a gas, making it easy to banish from a cell. (Mammals do it all the time.) If Earth organisms were silicon-based, however, they might have more of a problem, as silicon dioxide (SiO2) is a solid at room temperature.
A carbon backbone also ensures chemical processes necessary for life can occur more easily. For this reason (combined with a multitude of others), life on Earth uses a subset of elements: carbon, hydrogen, nitrogen, oxygen, phosphorous, and sulfur, also known as CHNOPS.
It’s entirely possible that some life can break the familiar CHNOPS paradigm. Scientists are looking for examples, but they’ve yet to find one on Earth. So, for now, any search for extraterrestrial life is focused on CHNOPS and governed by the rules of earthly chemistry. Other physical rules observed on Earth and in the solar system can also inform our search.
Follow the water
There’s another important ingredient for life: liquid. So far, all known life requires water. This makes sense — water helps move things around. It carries nutrients into cells, ferries waste away, and keeps things running smoothly. Whether life requires water for these processes is a key question in the search for life. Theoretically, any liquid could work.
Astronomers may be able to test this theory in our own cosmic backyard. Saturn’s moon Titan is absolutely frigid, with an average surface temperature of –290 degrees Fahrenheit (–179 degrees Celsius). Water is frozen solid at these temperatures. But other compounds, ones that usually exist as gases on Earth, are liquids. Both methane and ethane are prevalent on Titan and form clouds, rain onto the surface, flow into rivers, and pool into giant seas at the poles. Life could be hiding there.
If it is, it would be very different from all life on Earth, and not just because it wouldn’t use water. Liquids come in two distinct “flavors”: polar and nonpolar. Water is polar, meaning the H2O molecule has a positive end and a negative end. This is important because water only dissolves other polar molecules — like amino acids, proteins, or DNA — allowing cells to use them effectively. In contrast, methane and ethane are both nonpolar, so molecules that dissolve well in water will not dissolve in liquid methane or ethane. Thus, the complex molecules that Earth-based life depends on, such as DNA, would not be usable by any hypothetical life on Titan.
In our own backyard
With a whole universe of planets to explore, it may seem trivial to search for life within our solar system. But, unlike exoplanets, the worlds in our backyard are within reach. The nearest exoplanet is 4.2 light-years away. Even the fastest spacecraft humanity has launched would take nearly 20,000 years to reach it. In comparison, spacecraft can reach Titan and Europa in less than 10 years.
And there are even more worlds in our solar system to explore for life. The list so far includes Enceladus, Ceres, Ganymede, Callisto, Dione, Triton, and possibly even Pluto. Any one of these worlds could answer the question “Are we alone in the universe?” — albeit in a more microscopic way.
To paraphrase Carl Sagan, we stand at the shore of the cosmic ocean; recently, we have waded a little way out, and the water seems inviting. The more we search for life, the more we understand about our cosmic origins and the more questions emerge. But we have to search, because that is what makes us human: the drive to know, to learn, to discover. As Sagan so aptly puts it: “Hopefully, one day, we’ll realize that we are not alone in the cosmic dark, but that our pale blue dot is just one of many life-sustaining worlds scattered throughout the cosmos.”