This story comes from our special January 2021 issue, “The Beginning and the End of the Universe.” Click here to purchase the full issue.
Nearly 2,500 years ago, the Greek philosopher Democritus first proposed that objects are made of countless indivisible building blocks called atoms (Greek: atomos). However, it wasn’t until about 200 years ago, with the work of English chemist and physicist John Dalton, that the modern idea of atoms was developed.
Next came the challenge of learning to identify and distinguish between the various types of atoms. During the 19th century, advancements in spectroscopy — studying light by breaking it down into its constituent components — allowed scientists to discover that specific elements and molecules each have distinct spectral signatures. These signatures reveal themselves through unique combinations of emission and absorption lines (extra light and missing light, respectively) for each element. And by the mid-19th century, shortly after researchers first started classifying elements commonly found on Earth, astronomers began equipping their telescopes with spectroscopic sight to learn what the universe is really made of.
One of the obvious celestial targets for early spectroscopes was the Sun. When astronomers observed our star during a solar eclipse, with the Moon blocking most of the Sun’s overpowering light, they found a mysterious spectral line that didn’t correspond to any element yet known on Earth. The substance was dubbed helium, after the Greek word Helios, meaning Sun.
Early spectroscopic targets also included stars and planetary nebulae, but eventually, astronomers expanded their sights to include all astronomical objects. Today, we know from studying deep-sky (and, therefore, distant) targets that some common elements found on Earth have existed for almost the entirety of the cosmos’ life, created within the first minutes of the universe through the process of Big Bang nucleosynthesis (BBN).
Elemental origins
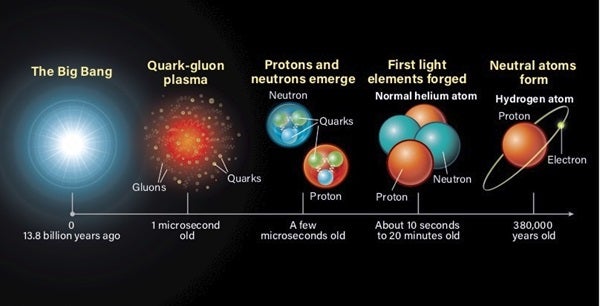
The universe didn’t create all of the elements at the same time, though. And each one has multiple pathways to formation. If we rewind to the very first moments of the universe, we find it was dominated by the smallest atomic building blocks — quarks, electrons, and other fundamental particles. Only later, a few millionths of a second after its birth, did the universe form protons (hydrogen) and neutrons as it rapidly expanded and cooled.
Soon after the first hydrogen formed, a couple of heavier elements quickly followed suit. But this process of BBN didn’t really kick off until the universe reached an age of just 10 seconds old. And it only lasted as long as 20 minutes.
Remarkably, the density of the universe at this time was incredibly low, about 100,000 times less dense than liquid water. If that’s the case, though, then why don’t we see nucleosynthesis occurring on Earth, where densities are much higher? The answer is that the temperature at that time of BBN was around 1 billion kelvins (1.8 billion degrees Fahrenheit, or just under 1 billion degrees Celsius). Thus, the earliest hydrogen atoms were zipping around so quickly that they frequently collided with great energy, which allowed them to merge into even heavier atoms like helium.
Within the universe’s first 20 minutes, it created most of the helium that exists today, as well as deuterium (heavy hydrogen) and a small amount of lithium. Over that same period of time, the ambient temperature of the universe dropped from about 1 billion kelvins to roughly 10 million kelvins, which is roughly the temperature found in the cores of stars, where stellar nucleosynthesis still occurs to this day. So, once the universe cooled down enough, BBN ceased producing the earliest and lightest elements.
Nonetheless, this early epoch saw so much helium created that the element ended up accounting for about 25 percent (by mass) of all the matter in the newborn universe. But astronomers want to know precisely how much of each element, particularly helium and deuterium, was produced during BBN. That’s because knowing these exact values is key to astronomers both confirming and better understanding the generally accepted theory for how the cosmos burst into existence: the Big Bang.
Forming helium and lithium
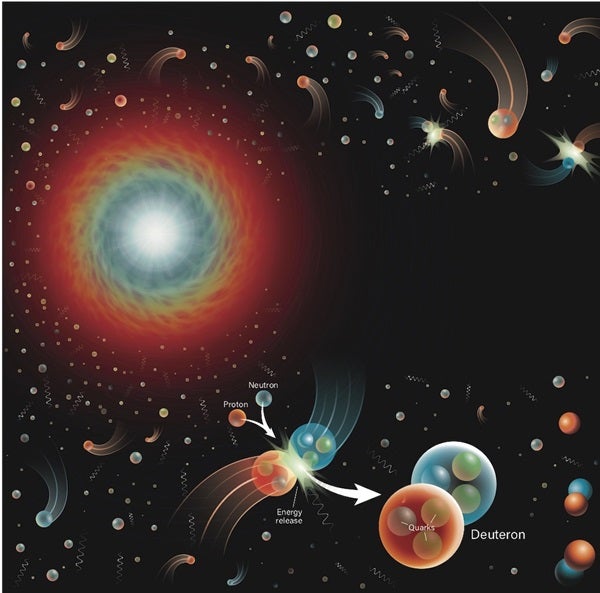
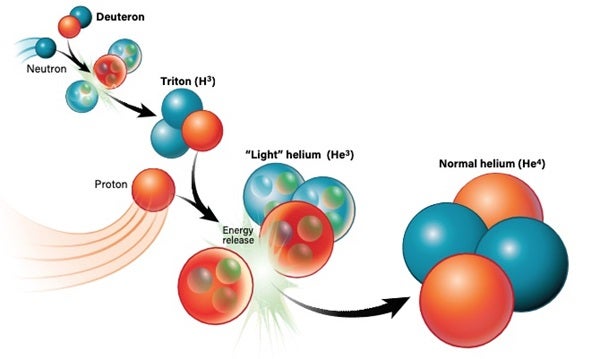
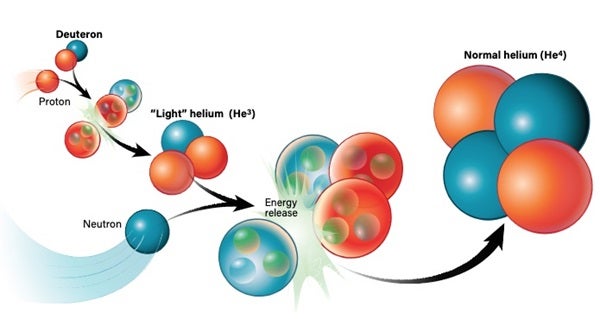
The nucleus of a regular hydrogen atom contains a single proton. But there’s a heftier version, deuterium, that can also exist. Deuterium is a hydrogen atom whose nucleus (a deuteron) contains a proton plus a neutron. So, although deuterium has the same charge as normal hydrogen, it’s about twice as massive. Deuterium is also relatively rare; on Earth, normal hydrogen is about 7,000 times more common than its heavier sibling. But once deuterium is around, it can go on to encourage the production of heavier elements like helium.
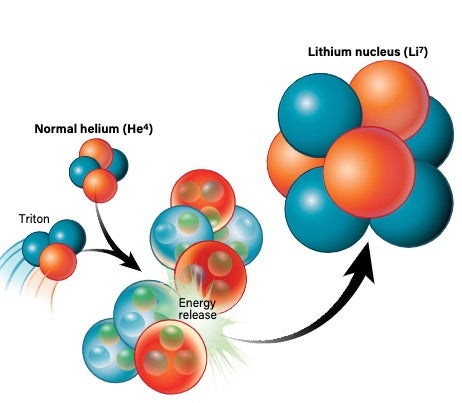
There’s more than one way to create helium. In the most basic sense, a deuteron and a neutron can join up to create tritium. By then adding another proton to the mix, you get a stable helium-4 atom. Alternatively, a deuteron and a proton can pair up to create a “light” version of helium called helium-3, which has two protons and one neutron. With the addition of another neutron, you get helium-4.
Similarly, if the expansion rate of the early universe were slower than theory predicts, then it would have remained in a dense state for a longer period of time, producing more light elements. If either of these things — matter density or expansion — were different than the Big Bang theory says, we would observe more helium than we see today. In other words: Primordial helium acts as one of many signatures notarizing the cosmos’ birth certificate.
Confirming our creation
It’s incredible that cosmologists can calculate the specific abundances of elements produced during BBN, then compare those predictions directly to the data. But, of course, one has to wonder whether our data really capture a pristine snapshot of the untainted abundances of elements that existed shortly after the Big Bang. Stellar nucleosynthesis surely leads to some contamination, but exactly how much remains an open and pressing question.
Fortunately, astronomers can observationally measure the abundances of important elements in a variety of ways. The most deterministic is to seek out deuterium and helium. This is often done by examining their prevalence in large pockets of quiescent gas around quasars, whose elemental compositions reveal themselves through absorption lines in their spectra. Because these gas clouds are largely devoid of stars, they’re expected to have experienced very little stellar evolution. They are ancient, relatively pristine cosmic relics. And when measuring the abundances of deuterium and helium in these clouds, astronomers find they line up with what Big Bang theory predicts.
This is largely seen as a wonderful confirmation of our universe’s origin story, as well as the entire process of Big Bang nucleosynthesis. But it’s also a fantastic example of how astronomy enables scientists to probe the very earliest moments of the universe, during the brief period of time when the first matter was being forged.