An atom becomes ionized when it absorbs enough energy to remove one or more electrons, which leaves a positively charged ion. The atom can acquire the energy necessary for ionization either by colliding with another particle or by absorbing radiation (light). The plasma state is, therefore, a “gas” of charged particles: positively charged ions and the negatively charged electrons that would be bound to the atoms if the system were in the ordinary gaseous state.
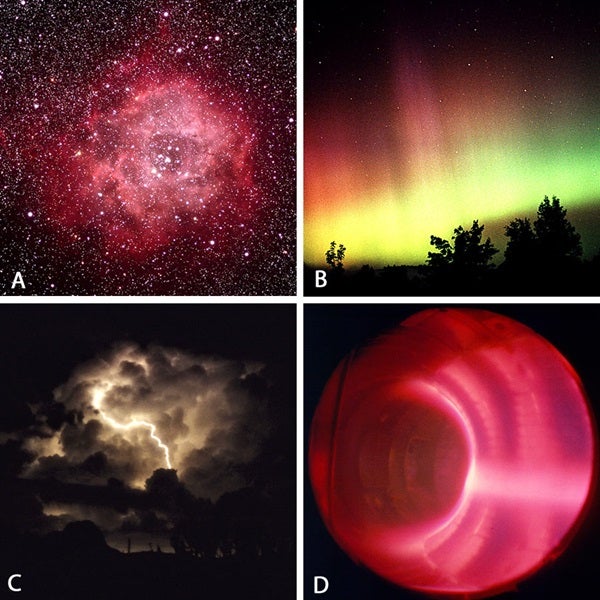
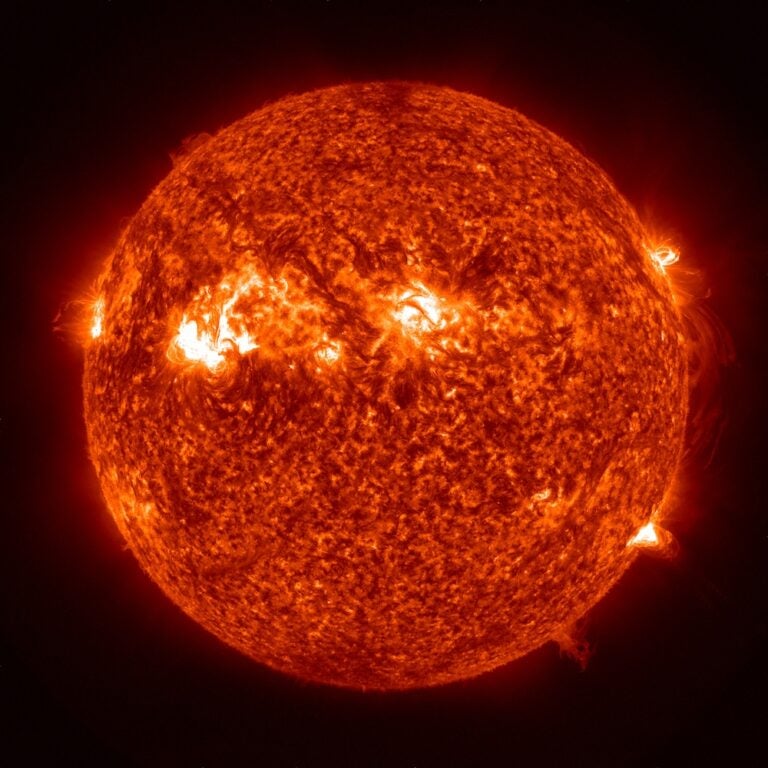
Because plasma is made up of charged particles, it can carry electric current and, therefore, produce and respond to electric and magnetic fields. A plasma state is ordinarily maintained only at temperatures greater than about 18,000° F (10,000° C). While the positive ions and the free electrons do recombine, frequent energetic collisions at these extreme temperatures tend to re-ionize any neutral atoms. At lower temperatures, recombination events occur more often than ionization events, so the state of the system is an ordinary neutral gas. High temperatures keep most of the matter in the core and atmosphere of the Sun and stars in the plasma state. Therefore, most of the ordinary (non-dark) matter in the universe is plasma.
Plasmas do not occur only in stars: Some nebulae, the solar wind, and the extended magnetic-field regions (magnetospheres) around planets like Earth and Jupiter are all plasma. The aurora borealis (or northern lights) provides evidence that plasma surrounds Earth. When charged particles from the magnetosphere stream into the upper atmosphere near the poles (where the magnetic field lines dive toward Earth), they collide with neutral gas atoms and cause the gas to emit light.
On Earth’s relatively cool surface, plasmas are usually human-made — lightning is one exception. Plasmas are artificially produced by passing an electric current through a low-pressure gas. Fluorescent lighting is the most common example of this.
Plasma TV screens display hundreds of thousands of pixels, and each is essentially a tiny fluorescent lightbulb that produces one of three colors (red, green, or blue). Because the plasmas in fluorescent bulbs (and plasma TV pixels) produce most of their light in the ultraviolet (UV) region of the spectrum, a phosphor coating on the inside of the fluorescent bulb converts the UV light to visible light. The pixels in a plasma TV have special phosphor coatings designed to produce red, green, or blue light.
Plasmas are also used in the production of semiconductor devices (such as computer chips) and in other manufacturing processes. Physicists also experimentally study plasmas. They trap and heat plasma with the hope of producing electric power by fusing the nuclei of heavy hydrogen (deuterium and tritium) into helium nuclei — the process that generates energy in the Sun and stars. — MATTHEW STONEKING, LAWRENCE UNIVERSITY, APPLETON, WISCONSIN