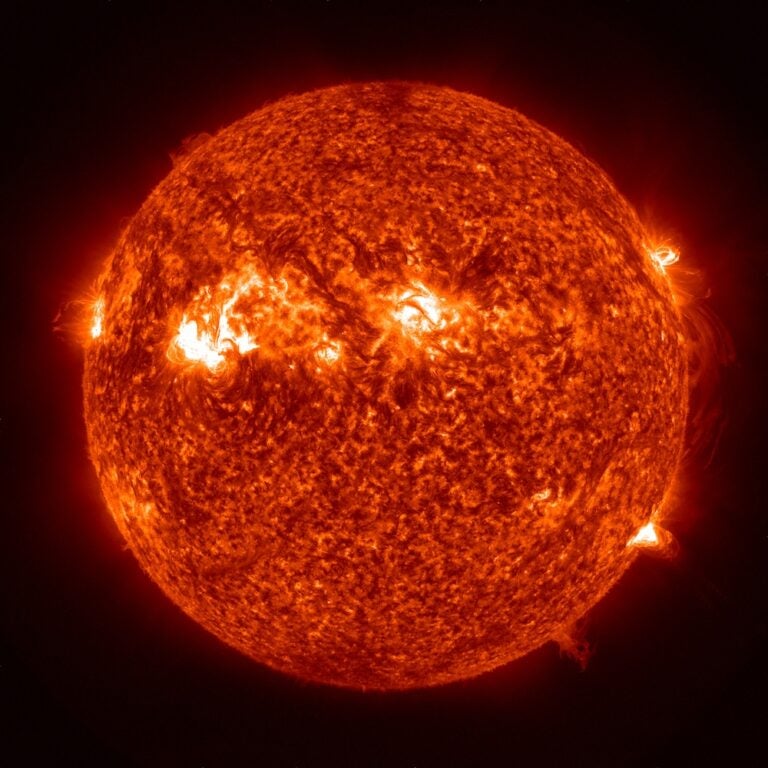
Q: Where does the iron in the Sun come from?
A: Observers commonly express the Sun’s composition by the percentage of total number of atoms. Ignoring the solar core, where hydrogen fuses to helium, the Sun’s outer layers consist of more than 91 percent hydrogen and more than 8 percent helium (all the former and most of the latter made within the first few minutes after the Big Bang). The rest of the chemical elements constitute just 0.15 percent or so of the number of atoms in the Sun.
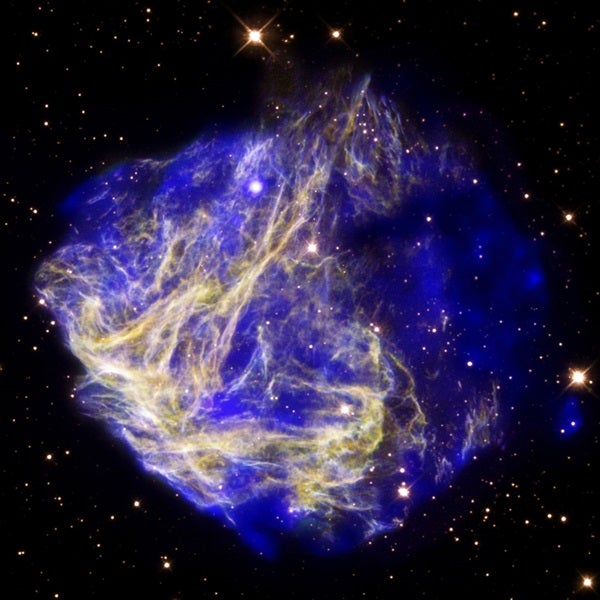
There is only one iron atom for every 31,600 of hydrogen. The Sun is not hot enough, even at its center, to make iron by the fusion of lighter elements. Instead, exploding stars, called supernovae, make all the iron strewn in the universe.
These explosions fall into two categories: In one, the core of a massive star suddenly collapses, while in the other, a Sun-like star’s remnant core — a white dwarf — becomes overloaded beyond its carrying capacity after drawing mass from a companion. The temperatures in the resulting explosions are so high that all the elements heavier than hydrogen, including iron, are created through fusion and other nuclear processes and then ejected into interstellar space. (While stars eight times as massive as the Sun create iron at their cores during their lives, that fused material collapses and evolves into a neutron star or black hole.)
The Sun is 4.6 billion years old, but our galaxy is 8 billion years older. The interstellar clouds from which the Sun formed had plenty of time to incorporate the iron and other heavy elements from supernovae into the infant Sun and its planetary system. The solar chemical composition, including its iron content, is vital information that we use to test theories of how stars and our galaxy evolved.
University of Illinois,
Urbana-Champaign